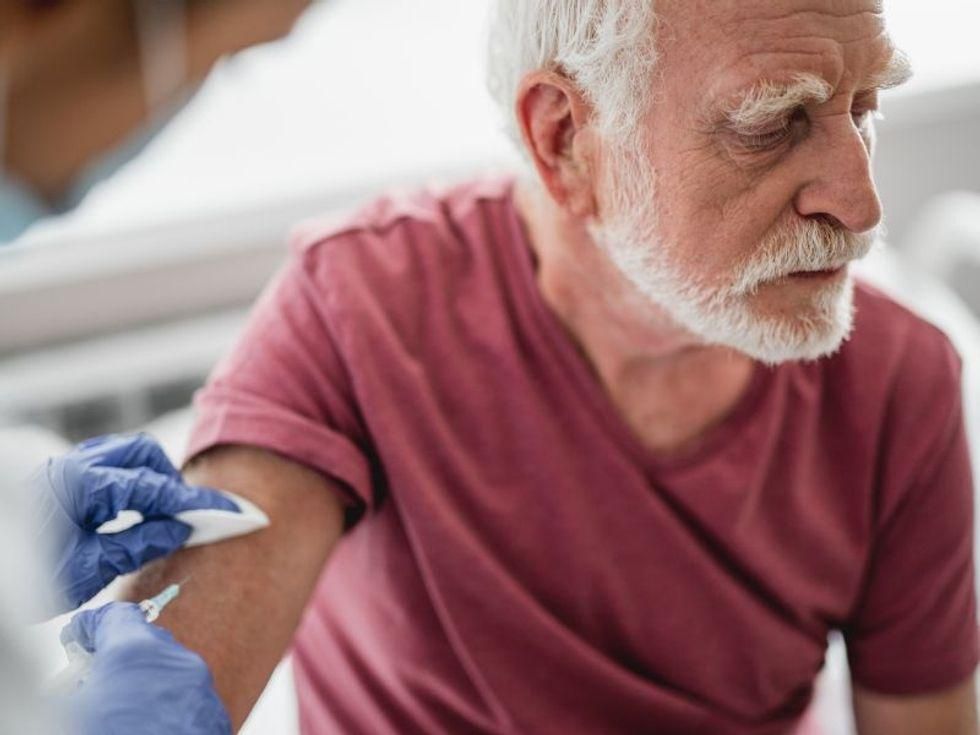
U.S. health experts warn there is a ticking time bomb in 11 states where 20 percent or more of seniors still haven’t gotten a COVID-19 vaccine.
Top priority for vaccinations was given to Americans aged 65 and older because they are far more vulnerable to serious illness and death from the virus than younger people are. Accordingly, this age group does have the highest rate of vaccination: 87 percent have received at least one dose, compared with 60 percent for people aged 18 to 64, and 31 percent for those aged 12 to 17, data from the U.S. Centers for Disease Control and Prevention show.
But in the 11 states where vaccination rates are lower among seniors, those who haven’t gotten a shot pose a public health risk as social distancing restrictions are stripped away.
Most of the 11 states are in the South: Alabama, Arkansas, Louisiana, Mississippi, North Carolina and Tennessee, The New York Times reported. Georgia, Idaho and Missouri are at the 20 percent threshold. West Virginia and Wyoming have more than 20 percent of people 65 and over without one dose.
“The 20 percent lines up pretty well with a group of people, especially in the South, who say, ‘No way, no how am I getting vaccinated,'” Dr. Michael Saag, associate dean for global health at the University of Alabama at Birmingham, told the Times.
“Convincing them that it is in their own interest is a tough nut to crack,” Saag noted. “For the state of Alabama and other Southern states, this is not for a lack of effort or resources. This is about a population resistant to receiving the message.”
Older people have felt more threatened from the coronavirus, experts say, so they have been among the most receptive to the vaccines. After older age groups were given priority when the first vaccines were authorized for emergency use in December, the proportion of those dying started dropping immediately, the Times reported.
Now, those aged 50 and older account for the bulk of COVID-19 deaths and the virus continues to kill hundreds of people daily. Death rates remain high in pockets of the nation where vaccination rates are low, the Times reported, and experts are concerned that these regions could face a surge in coronavirus cases over the summer.
“All epidemics are local at the end of the day, and transmission is person-to-person,” Saag told the Times. “There is going to be a hot pocket of transmission if someone becomes infected and others around them are unvaccinated. This is not Epidemiology 101, this is common sense.”
Last year, a summer surge lasted until September in the South. This year, many people are vaccinated and there is also residual immunity from those who have already had COVID-19, Saag said.
But the dropping of mask ordinances comes as the highly infectious Delta variant spreads, he noted. U.S. health officials this week classified the variant, first spotted in India, as a “variant of concern,” sounding the alarm because it spreads rapidly and may cause more serious illness in unvaccinated people.
“We’re sitting on a powder keg,” Saag said.
A Kaiser Family Foundation poll found last month that 10 percent of unvaccinated seniors said that they would “definitely not” get inoculated against the coronavirus. But the same poll showed signs that some hesitant people have been persuaded: About a third who had planned to “wait-and-see” on vaccination now say they are planning to get shots, the Times reported.
U.S. to spend $3.2 billion to help develop antiviral pills
After spending billions to speed the creation of COVID-19 vaccines, the United States said last week that it will now devote $3.2 billion to the development of antiviral pills that could stop the new coronavirus before it does its worst damage.
Along with “accelerating things that are already in progress” for COVID-19, the new program would also encourage treatments for other viruses, Dr. Anthony Fauci said when announcing the new program during a White House briefing on Thursday.
“There are few treatments that exist for many of the viruses that have pandemic potential,” he said, including Ebola, dengue, West Nile and Middle East respiratory syndrome.
But he added, “vaccines clearly remain the centerpiece of our arsenal.”
One antiviral drug, remdesivir, and three antibody therapies are now approved to treat COVID-19. But all of those drugs have to be delivered via IV at hospitals or medical clinics.
What is needed is a convenient pill that patients could take when symptoms first appear. Some drugmakers are testing such medications, but initial results aren’t expected for several more months, the Associated Press reported. The new federal funds will speed those tests and support research, development and manufacturing of the pills.
Last week, the United States said it would buy 1.7 million doses of an experimental antiviral pill from Merck and Ridgeback Biotherapeutics if it is shown to be safe and effective. A large study of the drug, molnupiravir, should deliver results this fall. Early research suggests the drug may reduce the risk of hospitalization if used shortly after infection, the AP reported.
The federal government may seek similar deals for two other antivirals that are in late trials, Dr. David Kessler, the chief science officer of the Biden administration’s COVID-19 response team, told the Times.
The hope “is that we can get an antiviral by the end of the fall that can help us close out this chapter of the epidemic,” Kessler said.
One of the drugs the government is eyeing is AT-527, developed by Atea Pharmaceuticals. The compound is already used to treat hepatitis C, and early studies suggested it might also work against COVID-19. Roche has partnered with Atea to test it in people, and the companies are currently running a late-stage clinical trial, the Times reported.
The other drug was created by Pfizer scientists, adapted from a molecule first designed in the early 2000s as a potential drug for SARS. After being abandoned for years, the Pfizer researchers decided to modify the molecule’s structure so it would work against the new coronavirus’s protease. More than 200 Pfizer scientists worked on the molecule, known as PF-07321332, the Times reported.
The drug had initially been designed to be taken intravenously, but the researchers tweaked its structure to work as a pill. When mice were given the pill, it reached high enough levels in the body to block the coronavirus. Pfizer launched a clinical trial in March to study its safety in people, and expects to move to later-stage testing next month, the Times said.
Until last week, the only medicines shown to boost survival were steroids given to patients sick enough to need extra oxygen and intensive care. But on Wednesday, U.K. researchers reported that one of the antibody combinations successfully reduced deaths in a large study of hospitalized COVID-19 patients, the AP reported.
More information
The U.S. Centers for Disease Control and Prevention has more on COVID-19 vaccinations.
SOURCE: Associated Press; The New York Times
Source: HealthDay

Leave a Reply