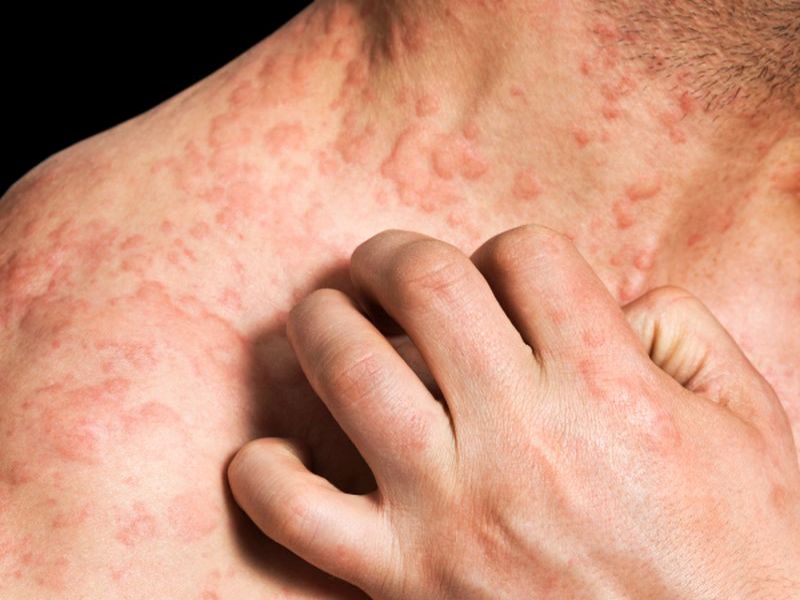
Adults plagued by eczema may have a new treatment option, with a new drug approved Tuesday by the U.S. Food and Drug Administration.
Dupixent (dupilumab) injections treat moderate-to-severe eczema in patients whose condition is not controlled by topical treatments or who should not use topical treatments. Eczema inflames the skin, making it red and itchy. It’s common in children, but can occur at any age and last a lifetime.
“Eczema can cause significant skin irritation and discomfort for patients, so it is important to have a variety of treatment options available to patients, including those patients whose disease is not controlled by topical therapies,” Dr. Julie Beitz said in an FDA news release. She is director of the Office of Drug Evaluation III in the FDA’s Center for Drug Evaluation and Research.
However, the drug is far from cheap. A year’s worth of the medication costs $37,000, The New York Times reported, although that price tag is still lower than biologic drugs that treat other skin diseases.
Dupixent can be used with or without topical corticosteroids.
One dermatologist welcomed Dupixent to the eczema arsenal.
“As science and technology and research has advanced our understanding of the pathways leading to chronic eczema, new and more defined treatments are becoming available and are very welcome,” said Dr. Doris Day, of Lenox Hill Hospital in New York City.
Day pointed out that these treatments “are easier to administer and give the patient a break from having to apply a cream over some or all of the body several times a day.”
The FDA approval is based on three clinical trials that included a total of just over 2,100 adults with moderate-to-severe eczema not adequately controlled by topical medications. After 16 weeks of treatment, those who received Dupixent had clearer skin and less itching than those who took an inactive placebo, the findings showed.
The drug can cause side effects, such as serious allergic reactions and eye problems, including pink eye (conjunctivitis) and inflammation of the cornea (keratitis). Patients who experience eye symptoms — such as redness, itching, pain or visual changes — while taking the drug should see a doctor, the FDA said.
In the clinical trials, the most common side effects of the drug included injection site reactions; cold sores in the mouth or on the lips; and eye and eyelid inflammation, including redness, swelling and itching.
The safety and effectiveness of Dupixent has not been established in those who are being treated for asthma, the FDA noted. Eczema patients who also have asthma should not adjust or stop their asthma treatment without talking to their doctors, the FDA added.
Dupixent is made by Regeneron Pharmaceuticals, based in Eastview, N.Y.
More information
The U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases has more on eczema.
Source: HealthDay

Leave a Reply