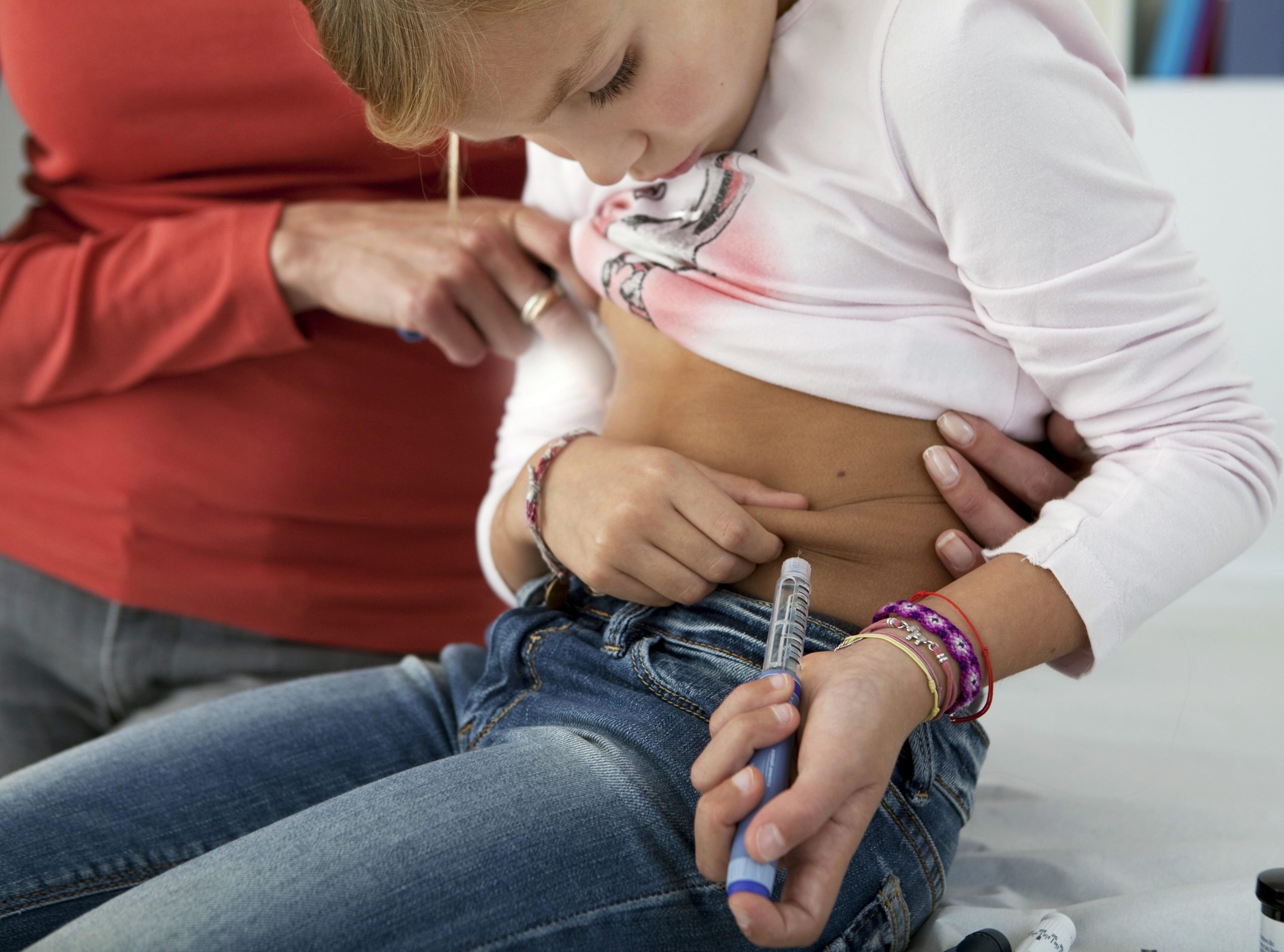
Science could be well on its way to a cure for type 1 diabetes, as researchers hone transplant therapies designed to restore patients’ ability to produce their own insulin, experts say.
At least one patient — a 64-year-old Ohio man named Brian Shelton — can now automatically control his insulin and blood sugar levels without the need for medication, following a transplant of experimental pancreatic stem cells.
Shelton’s therapy isn’t a perfect cure. He must take a heavy dose of immune-suppressing drugs to keep his body from rejecting the transplant, and those drugs pose their own health hazards.
But the therapy created by Vertex Pharmaceuticals could provide immediate relief to thousands who are lined up for a pancreas transplant because their type 1 diabetes has progressed to the point where it’s life-threatening, said Sanjoy Dutta, chief scientific officer for JDRF International.
Some of these folks are suffering hypoglycemic shock and landing in an emergency room multiple times a month, while others have developed resistance to the insulin shots or other diabetes medications that have helped keep them alive.
“Today, there are probably 5,000 to 10,000 people or more cued up for pancreatic or islet transplantation, but they’re not going to get it because there’s not enough supply,” Dutta said.
Researchers next plan to test Shelton’s first-generation cure on 17 people, to start gathering short- and long-term data on safety and effectiveness, said Dr. Yogish Kudva, a type 1 diabetes researcher with the Mayo Clinic in Rochester, Minn.
“The first goal is to do this at multiple centers. They’ve only reported one patient at this point,” he said. “They want to do 17 people and probably have the same approach in all 17.”
Vertex and other pharmaceutical companies are also surging forward to the next generation, looking at ways to further improve these stem cell therapies so they would require less immune suppression — or even none at all, Dutta and Kudva said.
Type 1 diabetes occurs when the body’s immune system turns against the cells in the pancreas that produce insulin, called beta cells. When enough beta cells have been destroyed, diabetic symptoms appear and can become severe in short order.
Thus, these stem cell transplants have to overcome not only the autoimmune disorder that caused type 1 diabetes, but also the immune response that causes the body to try to reject transplants as foreign invaders.
Two approaches under investigation involve coating the transplanted cells in a capsule that protects them from the immune system, experts said.
These capsules would allow nutrients and oxygen to come in and feed the cells, but prevent larger immune system cells from attacking the transplants, Dutta and Kudva said.
Unfortunately, a clinical trial by the pharmaceutical company ViaCyte involving fully encapsulated cells planted just under the skin did not prove successful, Kudva said.
A second approach by ViaCyte using partially encapsulated cells also didn’t go well, Kudva said. Insulin production didn’t become strong enough to provide benefit to the patients, who also needed immune-suppressing drugs to protect their now-vulnerable transplants.
“If you read the two papers, they did very little in terms of changing people’s insulin and changing people’s life,” Kudva said of the ViaCyte trials.
Companies haven’t given up on the capsule approach, however.
ViaCyte continues to tinker with its method, and Vertex has said that it plans to file an application with federal regulators this year to test its own encapsulated implant.
But ViaCyte is investigating a third way to protect the stem cell transplants — gene editing that would help the new beta cells evade detection by the immune system.
“You take the insulin-producing beta cells and you tinker with about seven, eight or nine genes,” Dutta said. “Tinkering with the genes will not compromise insulin production in any way, but it will render the cells cloaked or camouflaged from the immune system. You cheat the immune system to think these cells are not foreign.”
ViaCyte plans to start a clinical trial of a gene-edited stem cell therapy for type 1 diabetes by the end of the year, according to a statement from Aaron Kowalski, chief executive officer of JDRF.
It is “feverishly” supporting all three approaches to a cure, Dutta said.
“We have significant funding in the third bucket of gene editing and gene modification, because that’s where the future lies,” he said. “Which one will get to the finish line first, we don’t know. Which one will be the safest, we don’t know. Which one will be attainable for adult humans, we don’t know.”
It could be at least three to four years — maybe longer — before enough is known about the first-generation stem cell therapy to know whether it works long-term, much less make it available to the public, Kudva said.
“It’s going to be some years before we know where we are with this work, honestly speaking,” he said. “The first [patient] is good, but it was one. We have to do more. It’s going to take a little while here before we actually know.”
More information
The U.S. Centers for Disease Control and Prevention has more about type 1 diabetes.
SOURCES: Sanjoy Dutta, PhD, chief scientific officer, JDRF International (formerly known as the Juvenile Diabetes Research Foundation), New York City; Yogish Kudva, MBBS, type 1 diabetes researcher, Mayo Clinic, Rochester, Minn.; Aaron Kowalski, PhD, chief executive officer, JDRF, statement, Nov. 29, 2021
Source: HealthDay

Leave a Reply